The study of chemical reactions that include the movement of electrons is the focus of electrochemistry. Energy storage, corrosion protection, and biological systems are just a few of the many places it shines. The Nernst equation is a cornerstone of electrochemistry; it establishes a relationship between the cell potential of an electrochemical cell and the concentration of reactants and products. The purpose of this article is to provide a thorough introduction to the Nernst equation, including a discussion of its history, mathematical formulation, applications, limitations, and potential sources of error.
What is the Nernst Equation
In electrochemistry, the Nernst equation, developed by German physical chemist Walther Nernst, describes the relationship between the cell potential of an electrochemical cell and the concentrations of its reactants and products. As such, it can be used as a quantitative indicator of the electrochemical system’s equilibrium state.
Components of the Nernst Equation
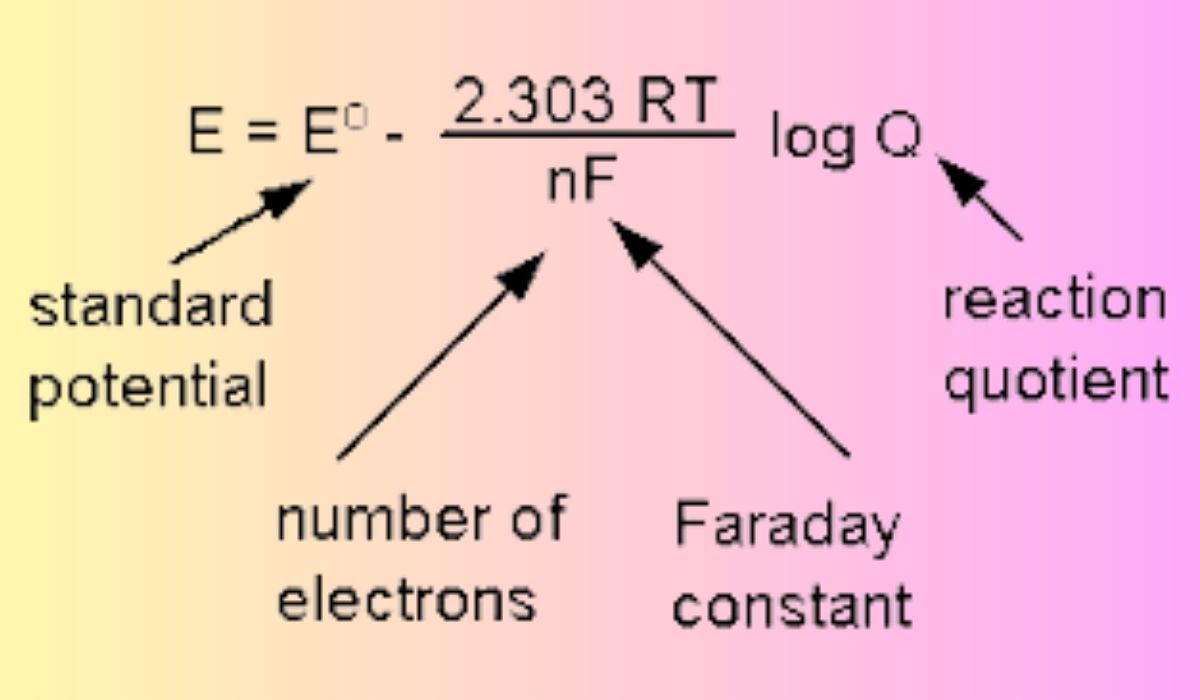
Standard electrode potential, reaction quotient, gas constant, and absolute temperature are the three basic ingredients in the Nernst equation.
Standard Electrode Potential
When all species concentrations are 1 M and gas pressure is 1 atm, the potential difference between an electrode and a reference electrode is known as the standard electrode potential (or standard reduction potential). It is used as a standard against which the electrode potential of half-cell responses can be evaluated.
Reaction Quotient (Q)
Q, short for “reaction quotient,” quantifies the ratio of reactant to product concentrations in a particular electrochemical system at a specific instant in time. It is found by dividing the reactant concentration by the product concentration, both of which have been adjusted to their stoichiometric coefficients.
Gas Constant and Absolute Temperature
As a fundamental constant in chemistry, the gas constant (denoted by the letter R) connects the energy scale of a system to its temperature. T stands for Kelvin (K), the unit of measure for absolute temperature. In the Nernst formula, RT is the product of the gas constant and the absolute temperature.
Understanding the Mathematical Formulation
Nernst Equation for Cell Potential
Cell potential is described by the Nernst equation, which is:
The cellular potential (Ecell) is calculated as follows: Ecell = (RT/nF) * ln(Q).
Where:
A cell’s potential, or Ecell,
The average cellular potential, or E°cell,
The constant of gas behavior is denoted by R.
The value of T is the absolute temperature.
n is the total number of electrons in a redox reaction of equilibrium.
Natural logarithm of the reaction quotient (ln(Q)) = F, where F is the Faraday constant.
Nernst Equation for Concentration
The cell potential can be determined from the concentrations of the reactants and products using the Nernst equation. It’s a result of:
Ecell = -E°cell * log(Q) / 0.0592/n
Where:
A cell’s potential, or Ecell,
The base-10 logarithm of the reaction quotient, Q, is denoted by the symbol “log(Q),” while “E°cell” stands for the standard cell potential and “n” for the number of electrons in the redox process.
Applications of the Nernst Equation
Numerous uses of the Nernst equations can be found in electrochemistry. Some prominent uses include:
Electrochemical Cells and Batteries
Understanding the operation of electrochemical cells and batteries requires familiarity with the Nernst equations. It aids in calculating the cell potential and making educated guesses about the likelihood and course of redox processes already under way.
pH Determination
Determining pH has close ties to the Nernst equation. The pH of a solution can be determined by monitoring the potential difference between a pH electrode and a reference electrode.
Potentiometric Sensors
The Nernst equation is essential to the functioning of potentiometric sensors, which are devices that measure the potential difference between an indication electrode and a reference electrode. These sensors are used in a wide variety of contexts, from biomedical devices and environmental monitoring to manufacturing.
Limitations of the Nernst Equation
The Nernst equation is a helpful tool in electrochemistry, although it does have some restrictions. For example:
Ideal Conditions: The equation is based on ideal conditions that may not hold true in practice.
Single Ion Activity: Ion activity coefficients in the solution are ignored, which can lead to inaccurate findings when using single-ion activity.
Concentration Dependence: For extremely concentrated or dilute solutions, the linear relationship between concentration and potential assumed by the Nernst equation may not be valid.
Factors Affecting Nernst Equation Accuracy
The precision of the Nernst equation is vulnerable to a number of potential errors. There are two main considerations:
Activity Coefficients
At high concentrations or in ionic solutions, the activity coefficients of ions in a solution might differ from unity. These inaccuracies make it necessary to modify the Nernst equation in some situations.
Deviations from Ideal Conditions
The Nernst equation is based on ideal conditions, which are typically not met in real-world electrochemical systems. Non-reversible reactions, side reactions, and temperature variations are just a few of the variables that can throw off the equation’s accuracy.
Conclusion
In conclusion, the Nernst equations is a useful tool in electrochemistry since it shows how the concentrations of reactants and products affect the cell potential of an electrochemical cell. It’s used for a wide variety of things, from storing energy to measuring pH to powering potentiometric sensors. However, its accuracy in real-world settings is affected by a number of aspects that must be taken into account. Insight into the behavior of electrochemical systems is gained, and progress is made in a number of scientific and technical fields, through familiarity with the Nernst equations.
FAQs
What is the Nernst equation used for?
To determine the equilibrium state of a system from its reactant and product concentrations, the Nernst equations is used to electrochemical cells to determine their cell potential.
Can the Nernst equation be applied to non-ideal systems?
Although the Nernst equations is based on ideal conditions, it can be adapted to non-ideal systems by taking into account non-ideal elements like activity coefficients and deviations from ideal conditions.
How is the Nernst equation related to pH?
Determining pH has close ties to the Nernst equations. pH can be determined with high precision using the Nernst equations and the potential difference between a pH electrode and a reference electrode.
Can the Nernst equations be used for gas-phase reactions?
It is for electrochemical cells in solution that the Nernst equations is most useful. Gas-phase reactions cannot be used as a direct analogy.
What is the significance of the Nernst equations in electrochemistry?
The Nernst equations plays a crucial role in electrochemistry because it enables the design and optimization of electrochemical devices and processes, as well as provides insights into the behavior of electrochemical systems.