The Pauli Exclusion Principle is a cornerstone of quantum physics and one of the most important rules guiding the subatomic behavior of matter. The renowned Austrian physicist Wolfgang Pauli formulated this concept in 1925, and it has since played a crucial role in our knowledge of the structure and behaviour of atoms, leading to a wide range of scientific advances. In this post, we’ll examine the Pauli Exclusion Principle in greater detail, diving into its significance, historical background, and practical applications.
The Genesis of Quantum Mechanics
The Pauli Exclusion Principle is best understood by going back to the beginning of the 20th century. The development of quantum mechanics was a watershed moment in the history of physics, ushering in a new era of research into the workings of the subatomic world. The probabilistic behavior and quantized energy levels of quantum theory called into question our deterministic worldview.
Wolfgang Pauli: The Mind Behind the Principle
The great physicist Wolfgang Pauli is central to the Pauli Exclusion Principles. Pauli, who was born in 1900, is often credited as the intellectual father of modern quantum physics. He proposed the exclusion principle because of his interest in quantum theory, and he ended up winning the Nobel Prize in Physics in 1945 for it.
Understanding the Pauli Exclusion Principle
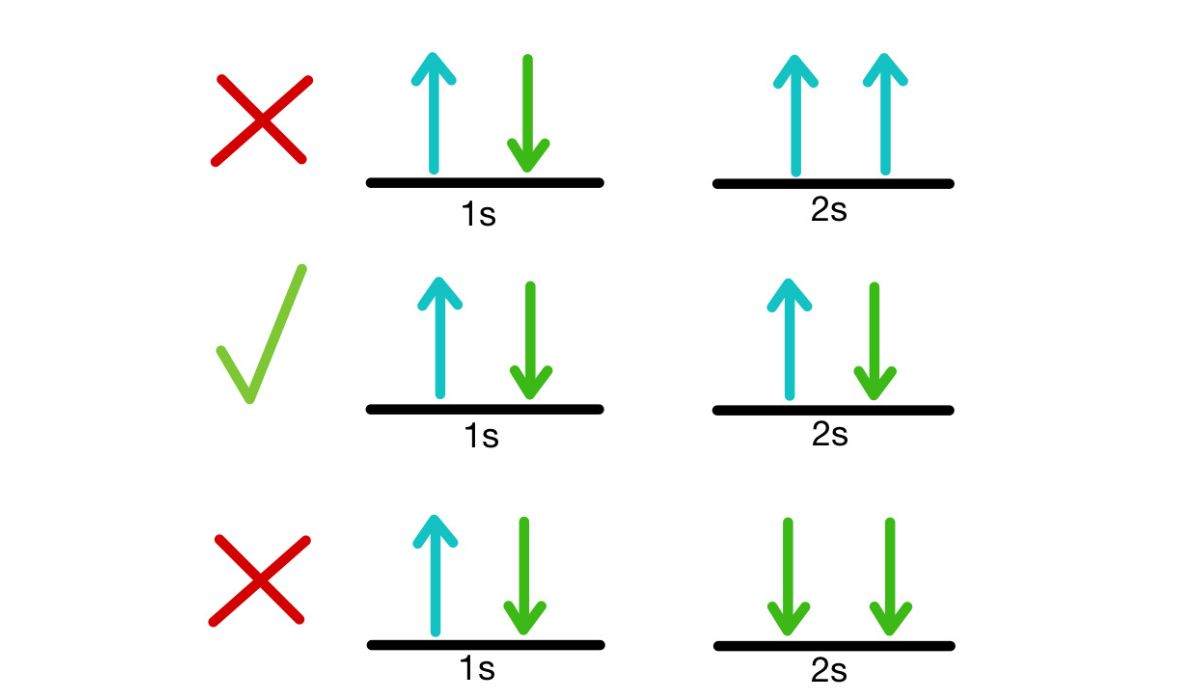
Since it is impossible for two identical fermions to be in the same quantum state at the same time, the Pauli Exclusion Principle states that they cannot be. Atoms’ complex electronic structures are the result of this principle being followed by fermions like electrons, protons, and neutrons. This phenomenon is crucial in establishing the chemical characteristics of elements.
Quantum Spin and the Role of Identical Particles
The idea of quantum spin is fundamental to the Pauli Exclusion Principles. Particles possess spin in addition to their intrinsic angular momentum. The importance of spin in explaining why similar particles are treated differently under the exclusion principle cannot be overstated.
The Impact on Atomic Structure
Atomic structure is substantially affected by the Pauli Exclusion Principles. It determines the characteristics and chemical reactivity of elements by controlling the placement of electrons in atomic orbitals. Matter as we know it would be impossible without the exclusion principle, and the very nature of our universe would be substantially different.
Exotic States of Matter
The Pauli Exclusion Principles has applications outside the realm of atomic and molecular physics, including in the realm of so-called exotic states of matter. Examples of the use of this principle can be seen in neutron stars, where the pressure of neutron degeneracy actually works against gravity.
Theoretical Advancements and Modern Research
The Pauli Exclusion Principle has fascinated physicists since its creation, and it shows no signs of slowing down. Current studies investigate its relevance to quantum computing and nanotechnology as well as its ties to other branches of physics, such as quantum entanglement.
Real-World Applications
The Pauli Exclusion Principle is often associated with the field of fundamental physics, but its implications extend well beyond this. This idea underpins much of modern technology and scientific discoveries, from semiconductors that power our electronic devices to the extraordinary insights afforded by spectroscopy.
The Pauli Exclusion Principle and Beyond
The Pauli Exclusion Principles has been with us the whole way on this exciting adventure into the quantum realm. Its far-reaching effects on physics and our comprehension of the cosmos continue to reveal new mysteries, luring us into the quantum realm we barely understand.
Conclusion
To sum up, the Pauli Exclusion Principle is still crucial to our understanding of the nature of atoms, matter, and the universe as a whole. Its importance in molding the observable physical universe cannot be emphasized. Future generations of scientists will no likely be inspired by the mystery of the quantum world to explore the world beyond our current understanding.
FAQs
What are the real-life implications of the Pauli Exclusion Principle?
The Pauli Exclusion Principles is fundamental to our understanding of the atomic structure, chemical characteristics, and even exotic states of matter, all of which have far-reaching practical ramifications.
Can the Pauli Exclusion Principle be violated?
The Pauli Exclusion Principles, a cornerstone of quantum physics, has endured the test of time without being disproved.
How does the Pauli Exclusion Principle affect electron configurations in atoms?
According to this concept, different elements have different electron configurations because no two electrons in an atom can share the same set of quantum numbers.
Is the Pauli Exclusion Principles related to the uncertainty principle?
Both the Pauli Exclusion Principles and the uncertainty principle are cornerstones of quantum physics, but whereas the former describes the behavior of identical particles, the latter describes the constraints placed on simultaneous measurements of specific attributes of a particle.
What is the connection between the Pauli Exclusion Principles and quantum entanglement?
Quantum entanglement is easiest to grasp when numerous identical particles are involved, as this situation violates the Pauli Exclusion Principles.