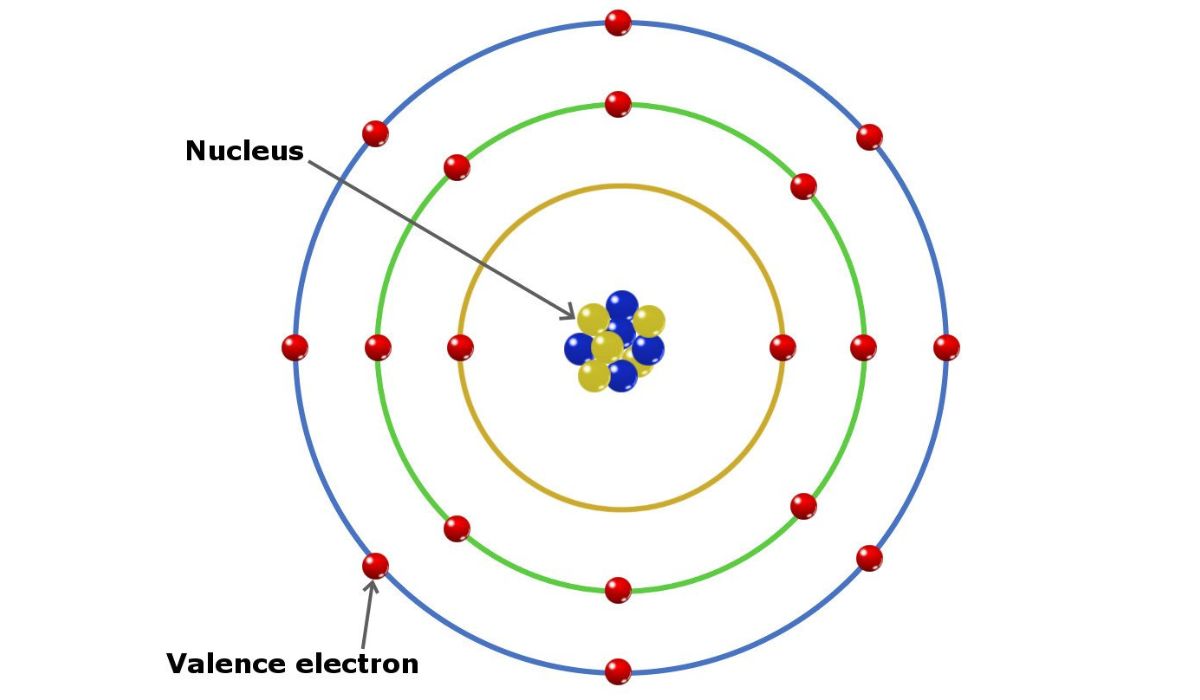
Valence electrons play a significant part in our understanding of atomic behavior and their significance in chemical reactions. The reactivity of an atom and its ability to form chemical connections with other atoms are determined by the electrons in its outermost shell, which are called valence electrons. The role of valence electrons in the periodic table and how they affect chemical bonding will be discussed in this article.
What are Valence Electrons
Atoms have valence electrons in their outermost energy level, also called a shell. These electrons are what cause an atom to react chemically with other atoms. The stability and reactivity of an atom, as well as its capacity to form various chemical bonds, are all affected by its valence electron count.
The Importance of Valence Electrons in Chemical Bonding
The significance of valence electrons in chemical bonding is crucial. They are the deciding factor in whether or not two atoms will form chemical bonds. In order to maintain a stable electron configuration, atoms may gain, lose, or share electrons, normally adhering to the octet rule. Atoms, according to this rule, should seek to have the same number of electrons at their outermost energy level as the noble gases, which is 8.
Valence Electrons and the Periodic Table
The number of valence electrons an element has can be determined by consulting the periodic table. A certain element’s position in the periodic table can provide light on its electron configuration and chemical properties. Elements with comparable valence electron configurations, as shown by their proximity on the periodic table, tend to share similar chemical characteristics.
Determining the Number of Valence Electrons
The location of an element in the periodic table can be used to infer the number of valence electron it possesses. The group number of a chemical element specifies how many electron its valence shell contains. Group 1 elements (hydrogen, lithium, etc.) have one valence electron, while Group 17 elements (fluorine, chlorine, etc.) have seven.
Octet Rule: Achieving Stability through Electron Configuration
Atoms will gain, lose, or trade electron until they reach a stable configuration with eight valence electron, as stated by the octet rule. By completing their outer electron shells, atoms achieve a state similar to the stable noble gases. Chemical bond formation is driven by the stability of electron configurations.
Elements with Unique Electron Configurations
While the octet arrangement is the goal of many elements, this is not always the case. Electron configurations are what set different elements like hydrogen, helium, and lithium. Hydrogen, which only needs two valence electrons for stability, and helium, which also only needs two valence electron but has a full outer electron shell, are both good examples.
Ionization and Valence Electron
Ionization occurs when electrons are added to or removed from an atom. The tendency of an atom to gain or lose electrons is strongly related to its valence electron count. Ions with positive charges are formed when an atom loses one or two of its valence electron, while ions with negative charges are formed when an atom gains one of its valence electron.
Valence Electrons and Chemical Reactivity
The number of valence electron in an atom is a major factor in the element’s reactivity. Elements with partial valence shells are less reactive than those with full shells because the former are always looking to gain electron. This phenomenon clarifies the common chemical characteristics shared by elements located in the same group of the periodic table.
The Role of Valence Electrons in Covalent Bonding
Covalent bonds involve the sharing of electron pairs between atoms in order to create a more stable electronic state. Sharing valence electron between atoms makes molecules more stable by allowing them to fill their outer electron shells. The power of a bond formed by sharing electrons is proportional to the number of electrons involved.
The Role of Valence Electrons in Ionic Bonding
Ionic bonding involves the sharing of valence electron between atoms to create ions with opposite charges. The process involves the donation of one or more valence electron from one atom, often a metal, to another, typically a nonmetal. An ionic bond is formed when two ions with opposite charges are attracted to one another.
Valence Electrons and Lewis Dot Structures
Valence electron can be shown graphically in Lewis dot structures of atoms and molecules. Each valence electron is denoted by a dot surrounding the atomic symbol in this nomenclature. The bonding and reactivity of an atom can be deduced from its Lewis dot structure.
Valence Electron in Transition Metals
Because of the presence of d orbitals, transition metals exhibit unusual electron configurations that break the octet rule. In certain metals, the number of valence electrons that contribute to bonds can change over time. The wide variety of chemical characteristics observed in transition metals is due in part to their capacity to create numerous oxidation states.
Valence Electrons in Nonmetals
It is easier for nonmetals to gain electron and generate negatively charged ions because they typically have a higher amount of valence electron. Nonmetals are able to establish covalent bonds and share electrons with other atoms because they have a higher electronegativity than metals.
Conclusion
Understanding the role of atoms and their valence electron in chemical reactions is crucial. Valence electrons play a crucial part in the synthesis of molecules and the variety of chemical compounds we perceive in the world around us by altering an atom’s reactivity and capacity to form chemical bonds.
FAQs
How do valence electron determine an element’s chemical properties?
The ability of an element to form chemical bonds is a function of its valence electron. Whether or not an element can create a certain type of bond depends on the number of valence electron it has.
Can elements have more than eight valence electrons?
Some elements, notably those in the third period and later, can have more than eight valence electron. The valence shells of these elements can grow to make room for more electron.
Are all chemical bonds formed by the transfer or sharing of valence electrons?
Hydrogen bonds are one type of chemical bond that do not involve the sharing or transfer of valence electrons. A hydrogen atom forms a bond with an electronegative atom due to electrostatic repulsion.
How do valence electrons contribute to the conductivity of metals?
Metals have delocalized valence electrons that are free to travel about the metal lattice. Metals are very conductive because their electrons are highly mobile.
What are the practical applications of understanding valence electron?
Material design, medication research, and the improvement of electronics and renewable energy technologies are just a few of the many real-world applications that can benefit from an understanding of valence electron.